What is ocean acidification?
Ever since the industrial revolution began over 200 years ago and humans began releasing large amounts of carbon dioxide (CO2) into the atmosphere, the ocean has been absorbing a large percentage (around 30%) of those carbon emissions. The ocean, as a carbon sink, has helped to slow the warming of the planet and is a large part of why the global increase in temperature has been so gradual; however, this carbon absorption has led to what is called ocean acidification. Ocean acidification refers to the increase of the acidity of the ocean as a result of human CO2 emissions, and this acidification can have significant effects on the marine environment and the organisms that live within it.
How does CO2 lead to an acidic ocean?
When carbon dioxide is absorbed by the ocean, it undergoes a chemical reaction with the water molecules, or H2O, that the ocean is made of. The CO2 and H2O react to form carbonic acid, or H2CO3; then, the carbonic acid breaks down into hydrogen ions, H+, and bicarbonate, HCO3-. This increase in hydrogen ions is what makes the ocean more acidic and lowers the pH of the marine environment.
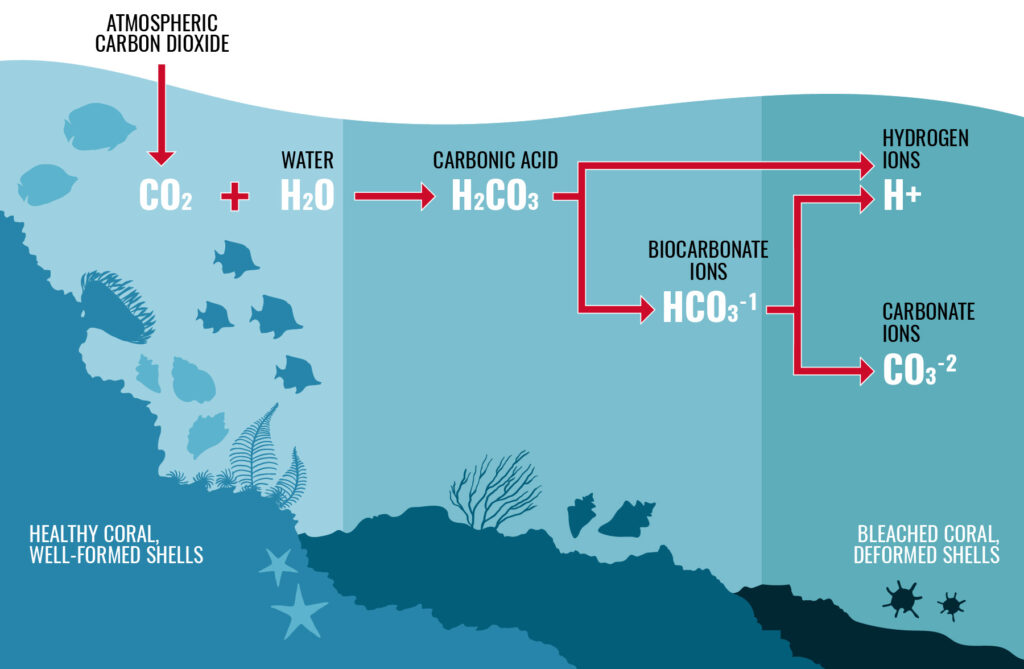
How does this affect shell-building organisms?
Many ocean organisms, including the mollusks that built the shells we see in the Cosman Shell Collection, pull a mixture of calcium, Ca(2+), and carbonate ions, CO3(2-), from seawater in order to create the hard shells they use as homes and as protection, which are made of calcium carbonate, or CaCO3 (for more detail on this, see How Mollusks Build Their Shells). However, when there is an increase in hydrogen ions (H+) in the water as caused by ocean acidification, the hydrogen ions bond to the carbonate ions in order to form even more bicarbonate. This means that there are less carbonate ions for shell-building organisms to pull from the water, which limits their ability to create the structurally sound shells that they need to survive. In addition, the existing shells of mollusks can begin to dissolve as the ocean seeks to restore chemical balance between carbonate ions and bicarbonate. The lack of carbonate building blocks and the dissolution of existing shells leave mollusks with weaker, thinner, and less organized shells, which affects their ability to survive in the ocean.

Learn More
Climate Interpreter on Ocean Acidification
Alliance for Climate Education on Ocean Acidification (Video)